
These electrons move from their ground states to higher energy. However, despiteĮlectrification the transitions are not very strong and a large column ofĬondensed H2 would be required, making it difficult to reconcile this Absorption spectra, in contrast, concern light frequencies of electrons that absorb energy. Possible to account for all of the DIBs with this one carrier. The absorption spectra of the system under investi-gation show the presence of isobestic points which indicate the existence of 1: 1 hydrogen bonded complexes between OH group of phenols and the lone pair of electrons on nitrogen atom.

Absorption spectra for hydrogen series#
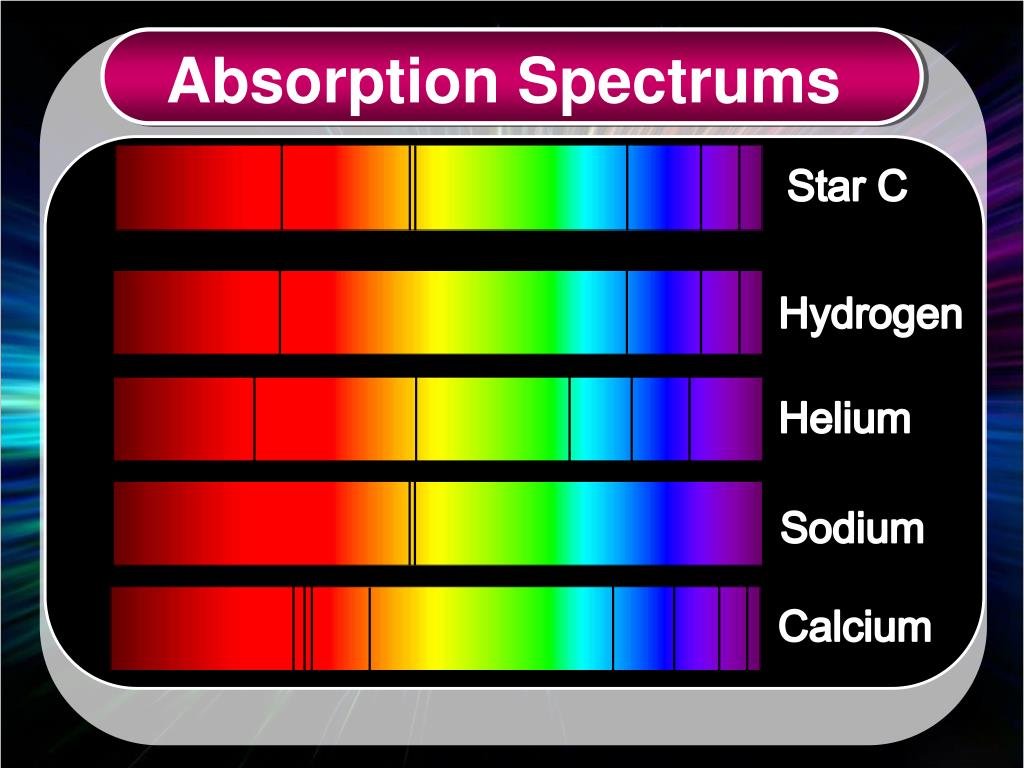
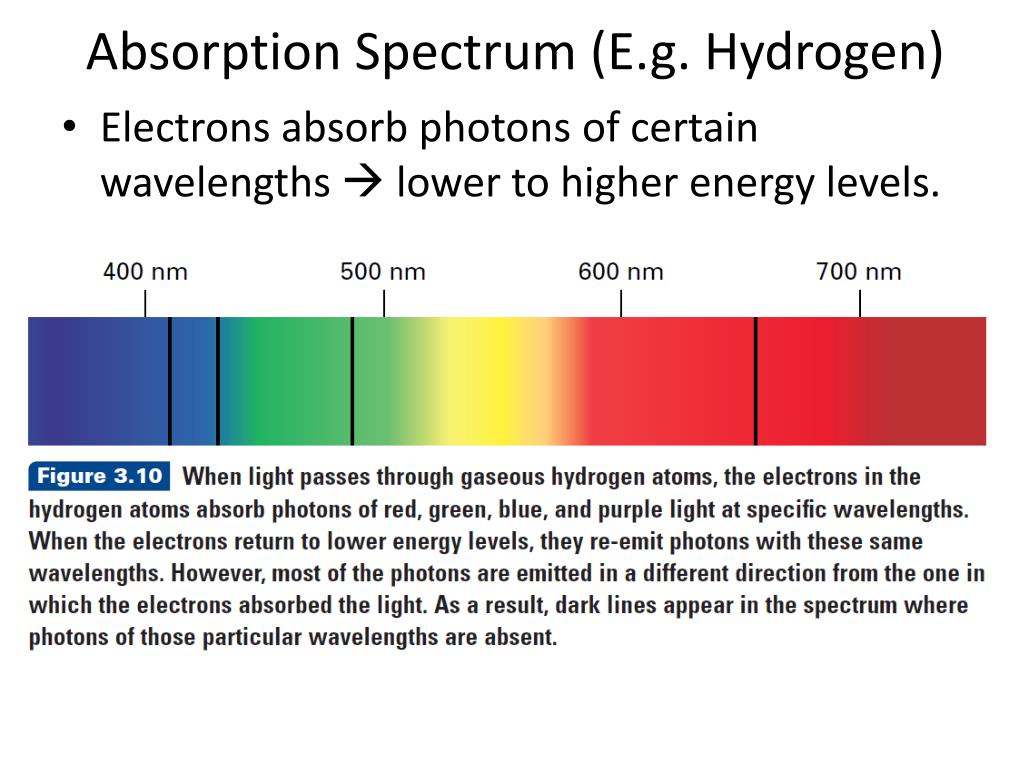
An absorption spectrum results when light from a continuous source passes through a cooler gas, consisting of a series of dark lines characteristic of the composition of the gas. We further argue that in principle it may be absorption region in the range 250-290 nm. An emission spectrum can be produced by a gas at low pressure excited by heat or by collisions with electrons. Therefore suggest electrified H2 as a possible carrier of the Diffuse Internal conversion, giving the absorption lines a diffuse appearance. Furthermore inĪ condensed environment the excited states likely have short lifetimes to Spectra are very different to that of the field-free molecule, so if theyĪppeared in astronomical data they would be difficult to assign. Hundreds of absorption lines across the optical and near infrared. Strongest amongst the states with high vibrational excitation, leading to Transitions that satisfy the dipole selection rules. The energy eigenstatesĪre mixtures of vibrational and angular momentum eigenstates so there are many Relevant to condensed hydrogen molecules in the interstellar medium: a uniformĮlectric field, and the field of a point-like charge. We restrict attention to two simple field configurations that are Here we use published ab initioĬalculations of the static electrical response tensors of the H2 molecule toĬonstruct the perturbed rovibrational eigensystem and its ground stateĪbsorptions.
Rovibrational states, but in a static electric field it acquires a dipole
Absorption spectra for hydrogen pdf#
Walker (Manly Astrophysics) Download PDF Abstract: Molecular hydrogen normally has only weak, quadrupole transitions between its The Hydrogen spectrum is an emission spectrum because the colored lines are separated by dark spaces, but the absorption spectrum has dark lines.Download a PDF of the paper titled Absorption spectra of electrified hydrogen molecules, by Mark A. Absorption spectrum is obtained when the white light if first passed through the substance and the transmitted light is analysed in the spectroscope. Emission spectrum is obtained when the radiation from the source is directly analysed in the spectroscope. The equation gives the calculation of the wave number ($\bar=\infty $. Several new peaks, of fairly general occurrence, are reported in the wavenumber range 3400 cm 1 to 2750 cm 1.

The Paschen series, Brackett series, and Pfund series belong to the infrared region.Īlthough a large number of lines are present in the hydrogen spectrum, Rydberg in 1890 gave a very simple theoretical equation for the calculation of the wavelength of these lines. The Balmer series belongs to the visible region. The Lyman series belongs to the ultraviolet region. The emission spectrum is similar except that in place of dark lines, there are colored lines with dark space in between. In absorption spectrum of hydrogen atom, only one electron is present in its one atom which is in ground state, so it means that all electrons can only absorb energy of photon of wavelength which lies in UV region to get to a higher energy state (by calculation it can take max wavelength 122.55 n m and minimum wavelength 91.9 n m ).Then why. The names of different series are the Lyman series, Balmer series, Paschen series, Brackett series, and Pfund series. It is found to consist of a large number of lines that are grouped into different series. The emission spectrum of hydrogen or line spectrum of hydrogen is produced when hydrogen gas is taken in the discharge tube and the light emitted on passing electric discharge at low pressure is examined with a spectroscope. There are 5 series in the hydrogen spectrum, Lyman, Balmer, Paschen, Brackett, and Pfund series. Hint: The line spectrum of hydrogen is also known as the Emission spectrum of hydrogen.


 0 kommentar(er)
0 kommentar(er)
